The discovery of the bacterium Helicobacter pylori (H. pylori) by Warren and Marshall in 1983 was a breakthrough in understanding the diseases of the stomach and duodenum. H. pylori is a curved, spiral gram-negative motile organism with 4 to 6 sheathed flagella.
The organism resides in or underneath the gastric mucus and is attached to the surface epithelium. It is a slow growing, microaerophilic bacterium, with some striking biochemical and biological features making it one of the most unique organisms in human biology.
The bacterium produces large amounts of urease which catalyzes the hydrolysis of urea present in the stomach to yield NH3 and CO2, thus elevating the pH to neutral as necessary for survival. H. pylori infects over half of the world’s population and is viewed as the most common human bacterial infection.
What are the diseases caused by H. pylori?
Pylori is ethologically associated with several gastroduodenal diseases. Over 90% of duodenal ulcers are caused by H. pylori infection and eradication of H. pylori in such patients through antibiotics prevents ulcer relapses.
Also, H. pylori eradication has been shown to prevent the recurrence of ulcer bleeds in patients with non-variceal upper GI bleeds and promote the healing of non-healing or giant duodenal and/or gastric ulcers. Kashmir is known to be highly endemic for peptic ulcer disease with a point prevalence of 4.72% and the lifetime prevalence was 11-22%.
The duodenal to gastric ulcer ratio is 17.1:1 [Khuroo et al. Gut 1989: 30; 930-4]. Majority of these are etiologically related to H. pylori in the community. There is intense debate about whether functional dyspepsia so prevalent in the community is associated with H. pylori infection.
H. pylori has no direct etiologic relationship with gastroesophageal reflux disease (GERD), however, GERD symptoms may worsen in case H. pylori-infected causes high acid secretion. Eradication of H. pylori in such cases can give a symptomatic improvement in such patients.
World Health Organization recognized H. pylori as a class Ⅰ [definite] carcinogen in 1994 and etiologically associated it with non-cardia gastric cancer [Adenocarcinoma intestinal type].
Gastric cancer is identified as the fifth most common malignancy and the third leading cause of cancer-related morbidity globally, constituting 9.7% of all cancer-related mortality. It is estimated that of the non-cardia gastric cancers, 89% are related to H. pylori bacterial infection and 9% are EBV related. Kashmir is an endemic zone for gastric cancer with age standardised incidence rates for men 36-7/100 000 per year and women 9.9/ 100 000 per annum (Khuroo et al. Gut, 1992, 33, 11-15).
Majority of these cancers are non-cardia, intestinal type of adenocarcinoma and ethologically related to H. pylori. Another cancer associated with H. pylori infection is gastric MALT lymphoma, a type of non-Hodgkin Lymphoma (12-18%) that occurs in about one in 100, 000 persons in the population.
Nearly all cases of gastric MALT are etiologically associated with H. pylori and short-term treatment with antibiotics to eradicate H. pylori infection cures gastric MALT lymphoma. Immune thrombocytopenia (ITP) and Iron deficiency anemia (IDA) are also associated with H. pylori infection.
ITP is an autoimmune disease mediated by anti-platelet autoantibodies. There is growing evidence that the eradication of H. pylori cures ITP by effectively increasing platelet count and disappearance of the anti-platelet autoantibody.
H. pylori infection is associated with IDA by impairing iron absorption as a result of chronic gastritis which causes gastric hypochlorhydria, leading to impair reduction of the dietary iron from the ferric to the ferrous form.
How do we contract H. pylori infection and what is its natural history in the human host?
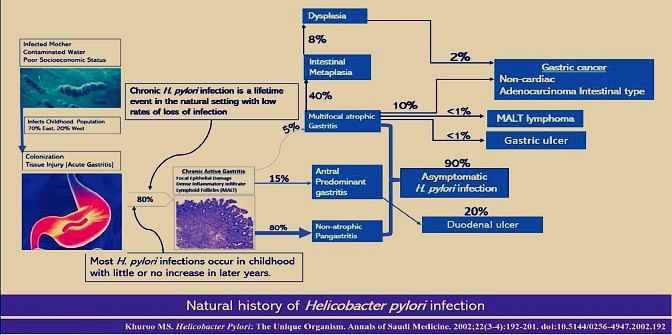
H. pylori is primarily a childhood infection, acquired orally either from infected mothers or contaminated water supplies supported by poor socio-economic conditions. The prevalence of infection is around 70% by 15 years of age in developing countries with little or no increase in later years.
In developed countries, there is a low prevalence (5-15%) in children with a high prevalence (20-65%) in the elderly population, presumably as a result of the cohort phenomenon. Once children get infected, over 80% develop chronic stomach infection, which is a lifetime event in the natural setting with low rates of infection loss.
H. pylori infection causes a characteristic syndrome of acute gastritis, which regresses over a period of a few weeks. Over 80% of patients develop chronic infection of the stomach causing chronic active gastritis. Most of these patients are asymptomatic, which is how H. pylori survive in the host. In fact, over 50% of the world’s population has this clinical setting. A small group of individuals could fall into two alternative tracts for reasons that are ill-understood at present.
About 5%-15% continue to have antral predominant gastritis with gastric hypersecretion and formation of duodenal ulcer. A smaller group shall develop a syndrome of multifocal atrophic gastritis with associated metaplasia and dysplasia, which eventually ends up in one of the three disease states, namely gastric ulcer, gastric cancer, and MALT lymphoma.
Q 4: What is the significance of the H. pylori genome? Ans: In 1997, the complete genomic sequence of H. pylori strain-26695 was published. H. pylori has a circular genome with over 16 hundred thousand base pairs and 1590 predicted coding sequences. Sequence analysis indicates that H. pylori have a well-developed system for motility, scavenging iron, and DNA restriction and modification.
Publication of the complete genomic sequence of H. pylori has marked the start of a new era of research into its role as a human pathogen. Several virulence factors, such as CagA and VacA, encoded by H. pylori genes, interact with gastric epithelial cells and the immune system, resulting in an inflammatory response, mucosal damage, and, eventually, gastric cancerogenesis.
Q 5: Who should be tested and treated for H. pylori? Ans: Testing for H. pylori is indicated and treatment in infected patients is beneficial in several disease states.
These include i. peptic ulcer (active or healed), ii. low-grad MALT lymphoma, iii. post gastric resection for early gastric cancer, iv. Immune thrombocytopenia, v. iron deficiency anemia, vi. chronic NSAID or low-dose aspirin therapy (to reduce ulcer bleed and improve compliance). When upper endoscopy is undertaken in patients with dyspepsia, gastric biopsies should be taken to evaluate for H. pylori infection, and treatment offered to those found infected.
In patients with uninvestigated dyspepsia without alarm features, nonendoscopic testing for H. pylori infection is a consideration. Patients with typical symptoms of GERD who do not have a history of ulcer disease need not be tested for H. pylori. If tested and found infected, treatment should be offered acknowledging that the effects on GERD symptoms are unpredictable.
There is insufficient evidence to support routine testing for and treatment of H. pylori in asymptomatic individuals with a family history of gastric cancer or prevention of gastric cancer, patients with lymphocytic gastritis, hyperplastic gastric polyps, hyperemesis gravidarum, and children with recurrent abdominal pain.
Q 6: How to test for H. pylori infection? Ans: The diagnosis of H. pylori can be done at upper GI endoscopy by rapid urease test and histological examination of gastric biopsies for organisms. The rapid urease test is very sensitive, but false-negative results can occur if the distribution of organisms within the stomach is patchy or if organism loads are low.
C13 or C14 urea breath test or Helic Ammonia breath test are global tests for H. pylori infection with high sensitivity and specificity. The stool antigen test detects the H. pylori protein antigen in the stools and is yet another sensitive and specific test for H pylori infection.
Serological tests are easy to perform but have limitations as they cannot differentiate current from past infections and are not recommended for diagnosis of H. pylori infection.
What are the treatment regimens for H. pylori and how to use them?
H. pylori eradication therapy is a matter of continuous debate with numerous drug combinations and a matter of confusion for a practitioner. However, several rules need to be followed in selecting a particular regimen which includes:
i. Use antibiotic combinations (2 or 3) as single agents give poor results, ii. Use a strong acid suppressant (PPI double dose) as it overcomes resistance to metronidazole and possibly other antibiotics, iii. Avoid metronidazole triple-based therapy if reported resistance rates in the community are high (>80%), as seen in our community.
iv. Avoid macrolide-based triple therapy if reported resistance rates are >15%, v. Previous exposure to macrolides should be taken into account while considering clarithromycin-based drug combination, vi. Amoxicillin therapy becomes an issue if there is a history of drug allergy and may need drug allergic testing, vii.
In case of a regimen failure, do not repeat any of the antibiotics in the second regimen, viii. As a general rule, 2 weeks of any regimen therapy gives better results. Some studies have shown similar results between 10 days versus 2 weeks of therapy. ix.
The main determinants of successful H. pylori eradication are the choice of regimen, the patient’s adherence to a multi-drug regimen with frequent side effects, and the sensitivity of the H. pylori strain to the combination of antibiotics administered.
Fig 2 gives an account of numerous regimens which a clinician has available as a first-line and rescue therapy. Use off these to treat primary and resistant H. pylori infections needs considerable experience, understanding and patience.
Should we test for treatment success after H. pylori eradication therapy?
Whenever H. pylori infection is identified and treated, testing to prove eradication should be performed using a urea or ammonia breath test, fecal antigen test, or biopsy-based testing at least 4 weeks after the completion of antibiotic therapy and after PPI therapy has been withheld for 1–2 weeks.
What methods can be used to evaluate for H. pylori antibiotic resistance and when should testing be performed?
Although H. pylori’s antimicrobial resistance can be determined by culture and/or molecular testing, these tests are currently not widely available routinely for clinical purposes.
How can we fight H. pylori globally?
There are two potential ways to fight H. pylori as a Class 1 human carcinogen and prevent the development of common and lethal non-cardia gastric cancer.
These include mass H. pylori eradication programs and the development of a vaccine. Long-term follow-up data from a randomized clinical trial carried out in Shandong China found that short-term treatment with antibiotics to eradicate H. pylori reduced the incidence of gastric cancer by 40% over a 15-year follow-up period. In recent years, a number of H. pylori vaccine development programs have been initiated.
All H. pylori vaccines currently in development are very early stages (Phase I or preclinical). These vaccines are predominantly composed of purified or recombinant components of H. pylori antigens with an adjuvant.
(PROF. MOHAMMAD SULTAN KHUROO MD, DM, FRCP (Edin), FACP, Master American College of Physicians (MACP, Emeritus). Former Director, Professor and Head Gastroenterology, Chairman Dept. Medicine, Sher-IKashmir Institute of Medical Sciences, Soura, Srinagar, Kashmir, India. Director, Digestive Diseases Centre, Dr.Khuroo’s Medical Clinic, Srinagar, Kashmir, India. E-mail: khuroo@yahoo.com, Website: www.drkhuroo.com.)
DISCLAIMER: The views and opinions expressed in this article are the personal opinions of the author.
The facts, analysis, assumptions and perspective appearing in the article do not reflect the views of GK.